Integrated expertise from concept to commercialisation. Our end-to-end services support every stage of cell and gene therapy development, ensuring regulatory success, process efficiency, and scalable manufacturing.
Exmoor Pharma
Our Services

Our services
Comprehensive Solutions for Cell & Gene Therapy
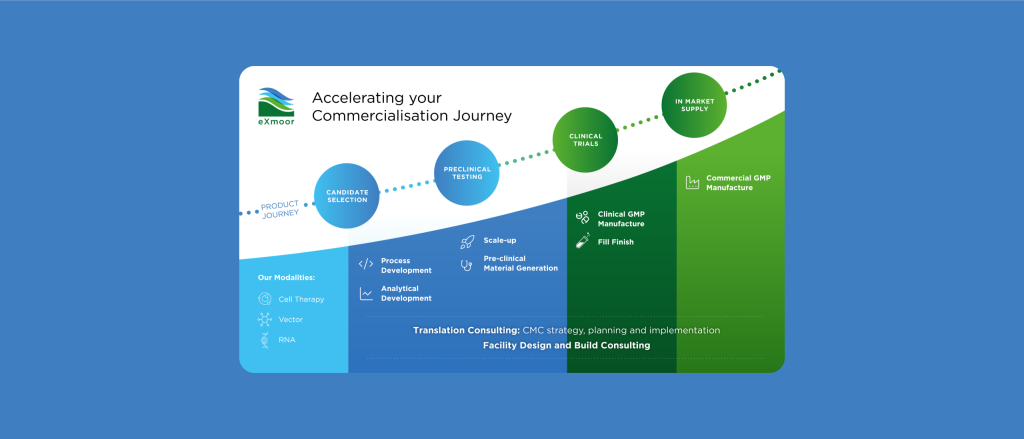
Bringing a therapy to market requires more than just manufacturing—it demands a strategic approach that integrates CMC strategy, process development, analytical services, GMP manufacturing and facility design.
With 20 years of experience, we provide tailored solutions to de-risk development, optimise processes, and enable successful commercialisation.
While our core expertise lies in cell and gene therapy, we’re always open to exploring new and emerging modalities. Many of the bioprocessing principles we apply are transferable across therapeutic platforms.
We thrive on challenging programmes and welcome the opportunity to support the development of novel therapies. If you’re working on something complex or novel, we’re always interested in hearing more.
Our Services
Consulting
Expert guidance to de-risk development and ensure regulatory success.
Process Development
Optimising and scaling CGT processes for seamless commercialisation.
Analytical Services
Generating high-quality data to ensure product safety, efficacy, and regulatory compliance.
GMP Manufacturing
Regulatory-compliant, flexible GMP manufacturing solutions tailored to your therapy’s needs.
Facility Design & Process Engineering
End-to-end facility planning, from concept to operational execution.
Testimonials
Case Studies
Explore case studies that demonstrate how our end-to-end capabilities help bring advanced therapies to patients faster.

Streamlining AAV Manufacturing for Clinical Scale-Up
Achieved GMP compliance through advanced optimisation, significantly enhancing yield and accelerating clinical readiness for a European biotech client’s AAV gene therapy.
Translating iPSC Processes for Scalable GMP Manufacturing
Successfully converted adherent iPSC cultures to scalable suspension systems, enabling GMP-compliant clinical manufacturing and commercial scale-up readiness.
Partner With Us
Whether you’re at the early stages of development or scaling for commercialisation, our expert team and cutting-edge facilities ensure your therapy’s success. Explore our services and discover how we can support your journey.
