Integrated expertise from concept to commercialisation. Our end-to-end services support every stage of cell and gene therapy development, ensuring regulatory success, process efficiency, and scalable manufacturing.
Exmoor Pharma
Our Services

Our services
Comprehensive Solutions for Cell & Gene Therapy
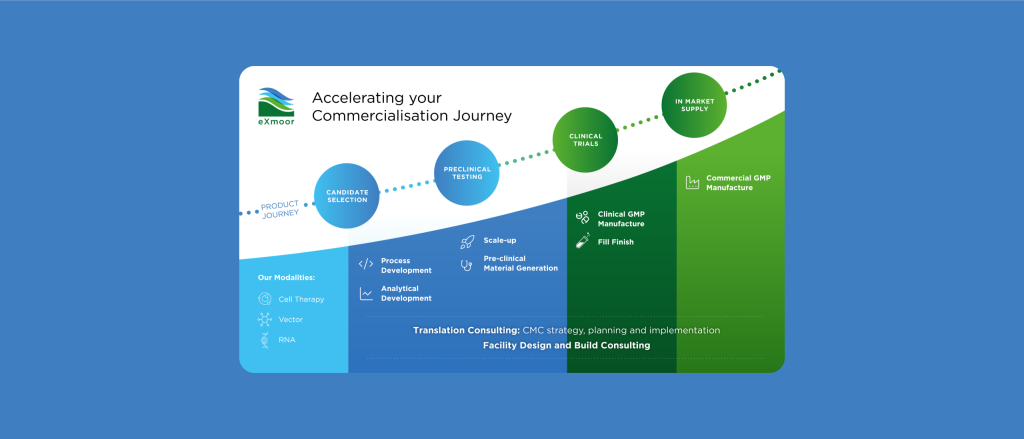
Bringing a therapy to market requires more than just manufacturing—it demands a strategic approach that integrates CMC strategy, process development, analytical services, GMP manufacturing and facility design.
With 20 years of experience, we provide tailored solutions to de-risk development, optimise processes, and enable successful commercialisation.
Our Services
Consulting
Expert guidance to de-risk development and ensure regulatory success.
Process Development
Optimising and scaling CGT processes for seamless commercialisation.
Analytical Services
Generating high-quality data to ensure product safety, efficacy, and regulatory compliance.
GMP Manufacturing
Regulatory-compliant, flexible GMP manufacturing solutions tailored to your therapy’s needs.
Facility Design & Process Engineering
End-to-end facility planning, from concept to operational execution.
Testimonials
Case Studies
Explore case studies that demonstrate how our end-to-end capabilities help bring advanced therapies to patients faster.

Streamlining AAV Manufacturing for Clinical Scale-Up
Achieved GMP compliance through advanced optimisation, significantly enhancing yield and accelerating clinical readiness for a European biotech client’s AAV gene therapy.
Translating iPSC Processes for Scalable GMP Manufacturing
Successfully converted adherent iPSC cultures to scalable suspension systems, enabling GMP-compliant clinical manufacturing and commercial scale-up readiness.
Partner With Us
Whether you’re at the early stages of development or scaling for commercialisation, our expert team and cutting-edge facilities ensure your therapy’s success. Explore our services and discover how we can support your journey.
