A well-defined CMC strategy accelerates cell and gene therapy development, ensuring regulatory compliance, manufacturing scalability, and commercial success. Build a robust approach with our expert guidance.
Consulting
CMC Consulting & Strategy

Why is a CMC Strategy Essential?
Developing cell and gene therapies is complex, requiring careful alignment of scientific, regulatory, and commercial considerations.
Without a strong CMC strategy, companies risk costly delays, regulatory setbacks, and manufacturing inefficiencies that can slow progress or derail development entirely.
A well-defined CMC strategy helps to overcome these challenges by ensuring regulatory alignment, manufacturing consistency, and economic viability at every stage.
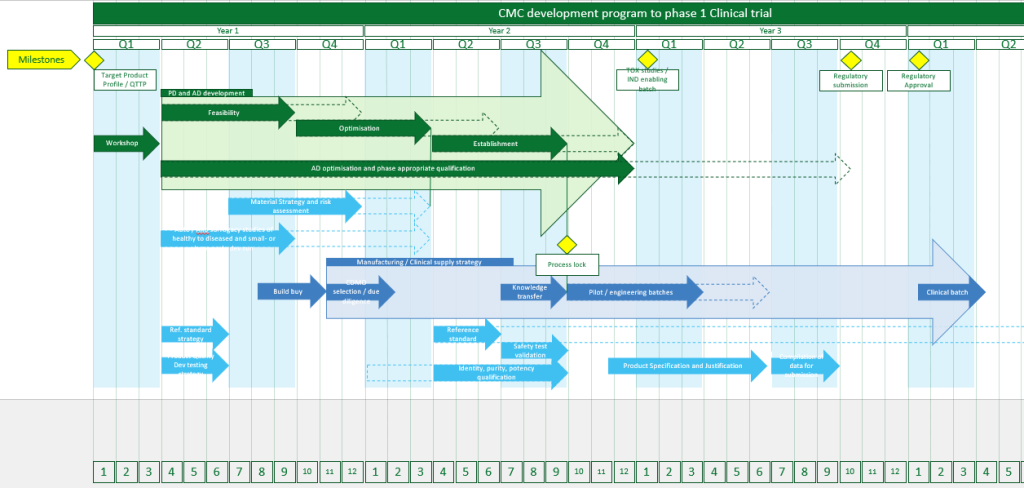
Our Process

Our Approach
We work closely with our clients to develop CMC strategies that are not only scientifically robust but also commercially viable.
Our CMC consultants work with biotech start-ups, established biopharma, and academic innovators, providing tailored guidance that ensures therapies are designed for success from the outset.
Our strategic approach focuses on four key areas that form the foundation of a successful CMC strategy.
Service Benefits
Quality Target Product Profile (QTPP)
Every therapy must meet both patient needs and regulatory expectations. We define critical product attributes early, ensuring that quality, safety, and efficacy are built into the development process.
Integrated Plans
A successful CMC strategy must align product development, manufacturing processes, and quality controls. We create tailored, end-to-end plans that anticipate market demands and regulatory requirements, providing a clear pathway to commercialisation.
Cost of Goods Analysis
Economic feasibility is essential for long-term success. We assess manufacturing costs, process efficiencies, and investment requirements to ensure that CGTs can be produced sustainably and at scale.
Risk-Based Framework
Identifying and mitigating risks early prevents costly setbacks. We help prioritise critical clinical, preclinical, and CMC considerations, ensuring that safety, product quality, and efficacy remain at the forefront of decision-making.
Key Deliverables
Lay the Groundwork for Success
A strong CMC strategy is the foundation of a successful therapy. Whether you’re advancing a novel cell therapy, scaling up for clinical trials, or preparing for commercial launch, we can help you create a roadmap that ensures efficiency, compliance, and long-term success. Contact us today to discuss how we can support your therapy’s journey.
