We deliver GMP manufacturing solutions that adapt to your needs, combining process-agnostic flexibility, regulatory expertise, and scalable production to advance cell and gene therapies from development to market.
Our Services
GMP Good Manufacturing Practices


Why GMP Manufacturing Matters
Bringing a cell or gene therapy to market requires a robust, scalable, and regulatory-compliant manufacturing strategy. Without Good Manufacturing Practice (GMP) assurance, companies risk delays, quality concerns, and regulatory setbacks that can jeopardise product approval and commercial success.
We provide flexible and process-agnostic GMP manufacturing solutions, designed to support biotech start-ups, established biopharma, and large pharmaceutical companies. Whether for early-phase clinical trials or commercial-scale production, our integrated manufacturing and regulatory expertise ensures a streamlined pathway to market.
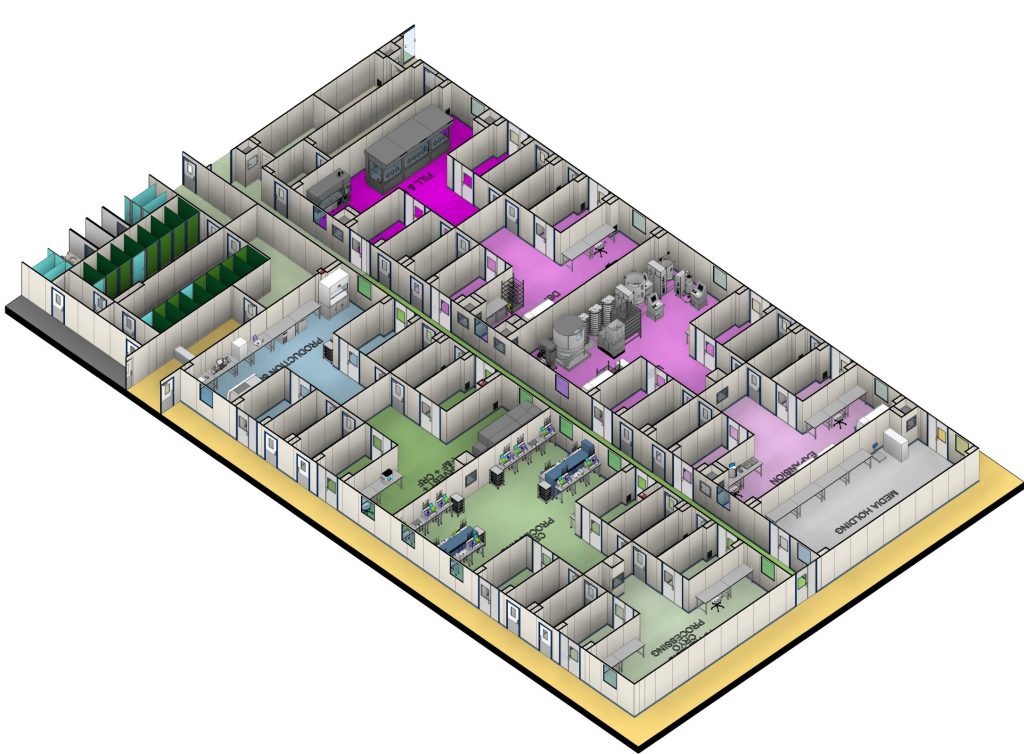
Our 65,000ft² (6,000m²) GMP facility in Bristol is purpose-built for cell and gene therapy manufacturing, offering:
- Four independent GMP clean rooms (Modules), supporting viral vectors, autologous, and allogeneic cell therapies.
- Grade C cleanroom environments, designed for closed processing with single-use disposables and Grade A conditions for open processing steps.
- Comprehensive Quality Control (QC) and Fill Finish capabilities, ensuring regulatory compliance and product integrity.
- Future expansion capacity of 12,000ft² (1,115m²), designed to accommodate clinical, pivotal, or commercial manufacturing for cell therapies, 500–1000L viral vectors, RNA, and LNPs.
GMP Manufacturing Services
Our Facility
Designed with flexibility, scalability, and compliance in mind, our GMP facility supports the full spectrum of cell and gene therapy modalities. Featuring dedicated manufacturing suites, Fill Finish areas, and QC laboratories, the facility ensures an end-to-end manufacturing solution under one roof.
Clinical Manufacturing
For companies in early-phase trials, our GMP clinical manufacturing capabilities provide scalable solutions with a focus on process robustness, regulatory compliance, and seamless technology transfer. Whether manufacturing autologous, allogeneic, or viral vector-based therapies, we offer the expertise and infrastructure to support successful clinical progression.
Commercial Manufacturing
With future-ready capacity and scalable GMP solutions, our commercial manufacturing services support clients moving into late-stage and commercial production. Our 12,000ft² (1,100m²) expansion space is designed to accommodate larger batch production, supporting 500–1000L viral vector processes, RNA, Lipid Nanoparticles (LNPs), and other advanced technologies.
Cell Banking
We offer GMP-compliant cell bank production as a standalone service or as part of a wider manufacturing programme. With experience in banking iPSCs, MSCs, and HEK293s, our services include 2D/3D expansion, validated cryopreservation, secure storage, and full QC testing and documentation.
Fill Finish
Our dedicated fill finish module includes Grade C cleanroom space and a closed Grade A isolator system. We support clinical vial and bag filling for viral vectors and allogeneic therapies, offering standalone or integrated fill finish with full QA oversight and sterility assurance.
GMP Quality & Regulatory
Our in-house QPs and regulatory specialists offer proactive quality oversight and GMP support, from documentation and batch release to audit readiness and training. We ensure compliance is embedded at every stage of your manufacturing journey.
Why Partner with eXmoor Pharma?
Let’s Build Your GMP Manufacturing Strategy
Whether you require clinical trial material, commercial supply, or manufacturing scale-up, we provide the expertise, facilities, and regulatory confidence to move your therapy forward. Contact us today to discuss your GMP manufacturing needs.
