Purpose-built for flexibility, scalability, and regulatory excellence. Our GMP facility provides a process-agnostic, sustainable environment to support clinical and commercial manufacturing.
GMP Manufacturing
Our Facility

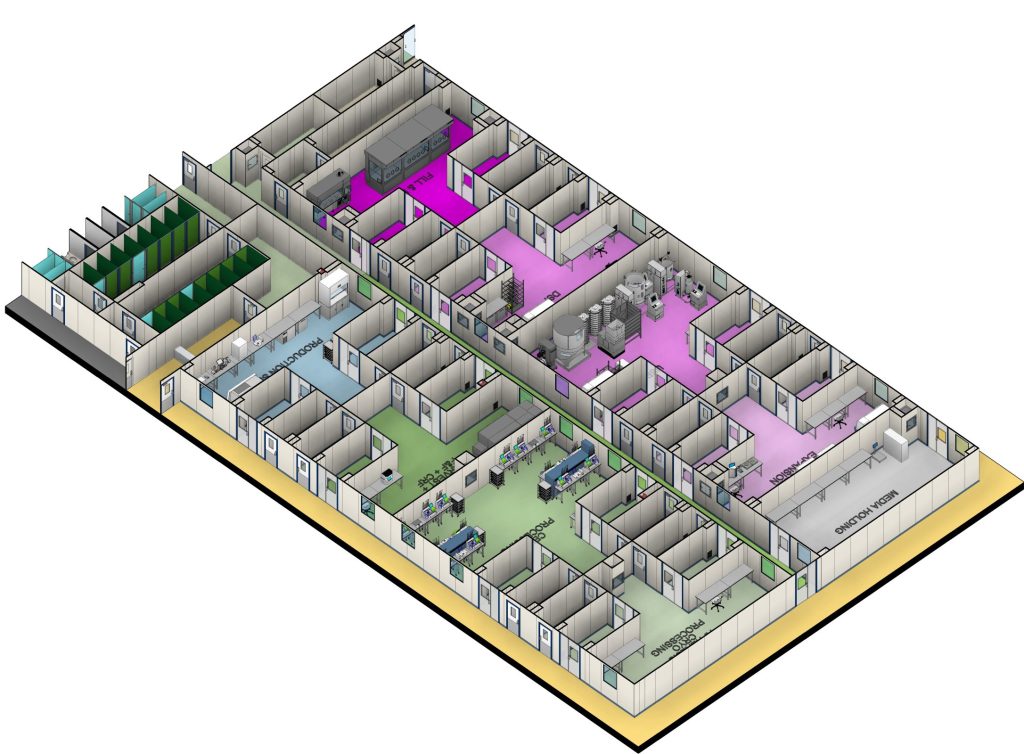
A Flexible, Scalable, and Sustainable GMP Facility
Bringing a cell or gene therapy to market requires a GMP-compliant manufacturing environment that supports efficient scale-up, process adaptability, and long-term commercial viability. Without the right infrastructure, companies face regulatory hurdles, operational inefficiencies, and production constraints.
Our 65,000ft² (6,000m²) GMP facility is designed to be flexible, process-agnostic, and scalable, ensuring we can support autologous and allogeneic cell therapy, viral vector, and RNA-based manufacturing.
With four fully qualified and operational Grade C cleanrooms, cutting-edge process capabilities, and a range of GMP-compliant storage solutions, including ambient, refrigerated, and deep-freeze options, we provide a seamless transition from process development to GMP production.
Sustainability is embedded throughout our facility, reducing energy consumption and operational costs while maintaining the highest regulatory standards.
Facility Capabilities
Why Partner with Us?
Experience Our Facility Firsthand
Discover how our state-of-the-art GMP facility can support your clinical and commercial manufacturing needs. Contact us for a tour and explore the possibilities.
