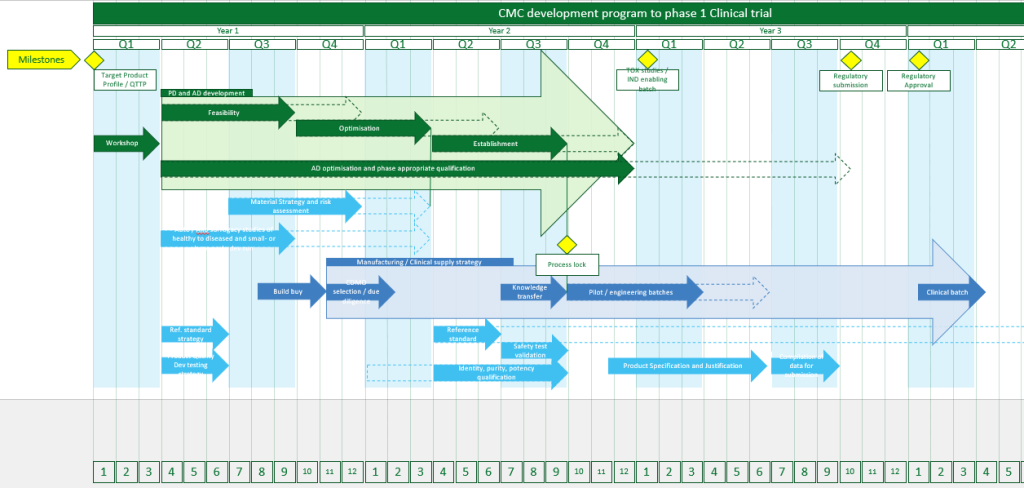
Developing iPSC-based therapies demands more than scientific ambition. It requires a strategic, technically rigorous approach that can carry you from early-stage research to GMP manufacture.
That’s where we come in.
We partner with iPSC developers to translate research protocols into robust, regulatory-aligned processes. Whether you’re working with early platform lines or navigating multiple differentiation pathways, our integrated team helps define your CMC strategy, optimise development, and deliver clinical material with confidence.