Developed a strategic CMC roadmap for Tavira’s AAV-targeting platform, aligning internal teams and investors around clear priorities for clinical development.
Case Studies
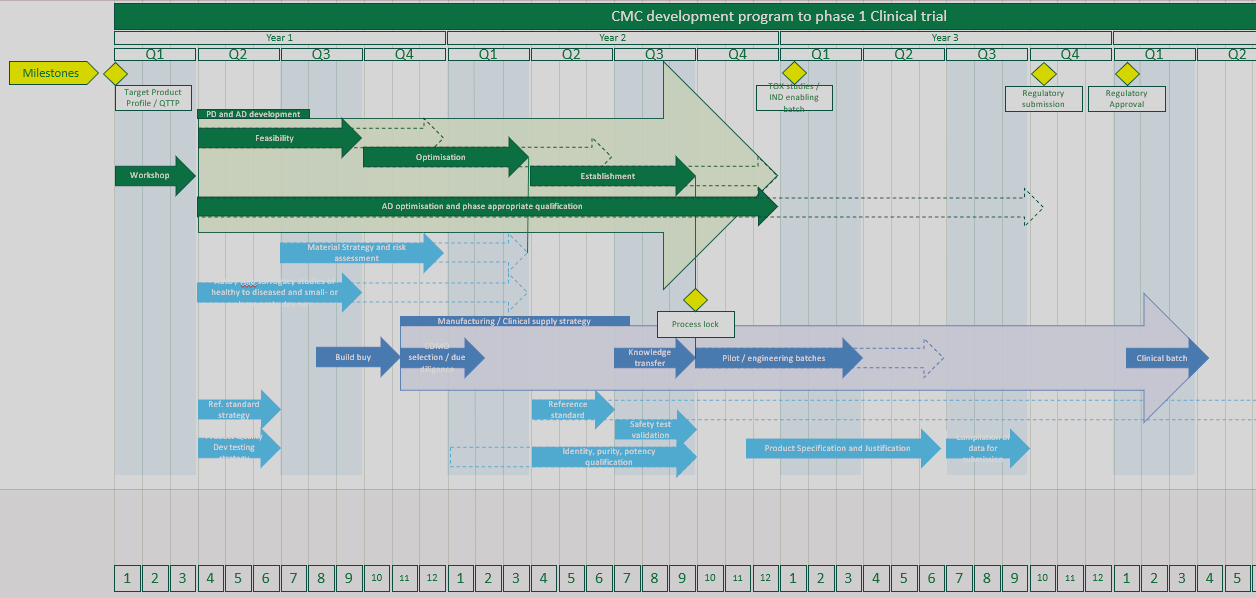
Building a CMC Roadmap for an AAV-targeting Platform
The Challenge: Establishing CMC for a New Platform
Tavira Tx, a biotech developing an AAV-targeting platform, needed to define a clear CMC strategy to progress towards clinical asset development. The company sought expert support to assess manufacturability and reproducibility, establish quality considerations across its portfolio, and understand the regulatory requirements at this stage.
Our Approach
eXmoor Pharma structured a three-phase consulting project. We began with workshops to assess Tavira’s platform, manufacturing processes and portfolio priorities. Findings were consolidated into a detailed CMC strategy report, which was reviewed and refined with the client team. A final alignment session set out next steps for development and regulatory preparation.
Deliverables
The Outcome
Impact

The engagement gave Tavira clarity on its development path, providing an actionable roadmap that aligned its leadership team and investors around key priorities. By combining scientific insight with regulatory and quality foresight, eXmoor created a practical plan that enables Tavira to progress its platform toward clinical asset development with confidence.

“eXmoor Pharma helped us translate our scientific platform into a clear, phase-appropriate CMC plan. Their structured approach identified the critical challenges and set out practical next steps toward first-in-human studies, giving our team and investors confidence in the path ahead.”
Case Studies
See how eXmoor Pharma has helped biotech innovators scale their therapies with precision and efficiency.
Optimising AAV8 Manufacturing for Preclinical Success
Improved scalability and purity of AAV8 processes, providing high-quality material and accelerating clients’ path towards successful clinical trials.

Streamlining AAV Manufacturing for Clinical Scale-Up
Achieved GMP compliance through advanced optimisation, significantly enhancing yield and accelerating clinical readiness for a European biotech client’s AAV gene therapy.
Let’s Accelerate Your Therapy Together
Whether you’re in early-stage development or scaling up for commercial production, eXmoor Pharma provides the expertise and flexibility you need. Partner with us to move your therapy forward with confidence.
